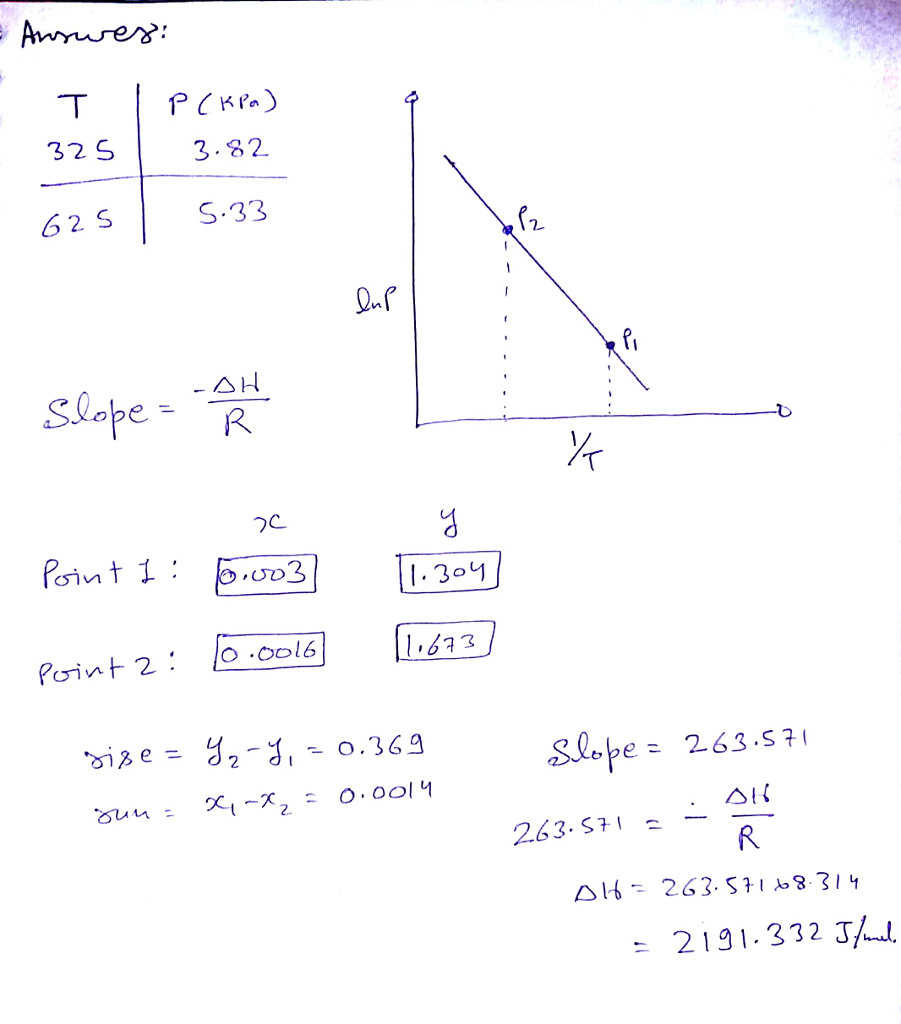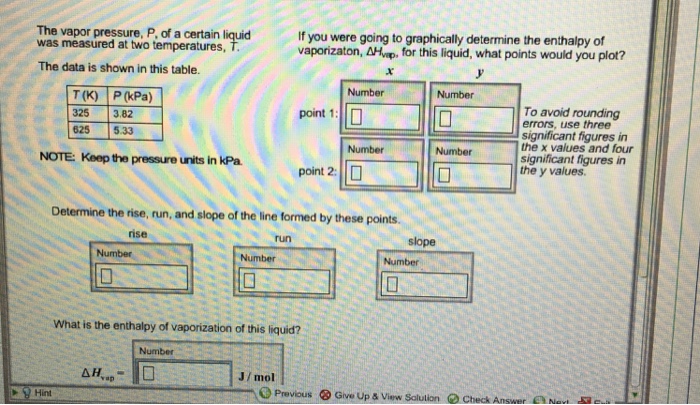
The vapor pressure, P, of a certain liquid was measured at two temperatures, The data is shown in this table. Determine the rise, run, and slope of the line formed by these points. What is the enthalpy of vaporization of this liquid? Delta H_ = If you were going to graphically determine the enthalpy of vaporizaton, Delta H_ for this liquid, what points would you plot? To avoid rounding errors, use three significant figures in the x values and four significant figures in the y values.