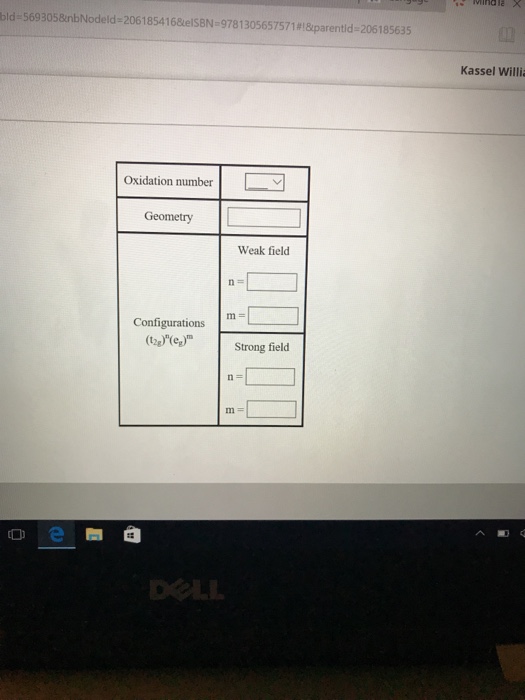
The compound [Co(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of cobalt in this compound, the geometry of the coordination around the cobalt, and the possible configuration of the d electrons of the cobalt.

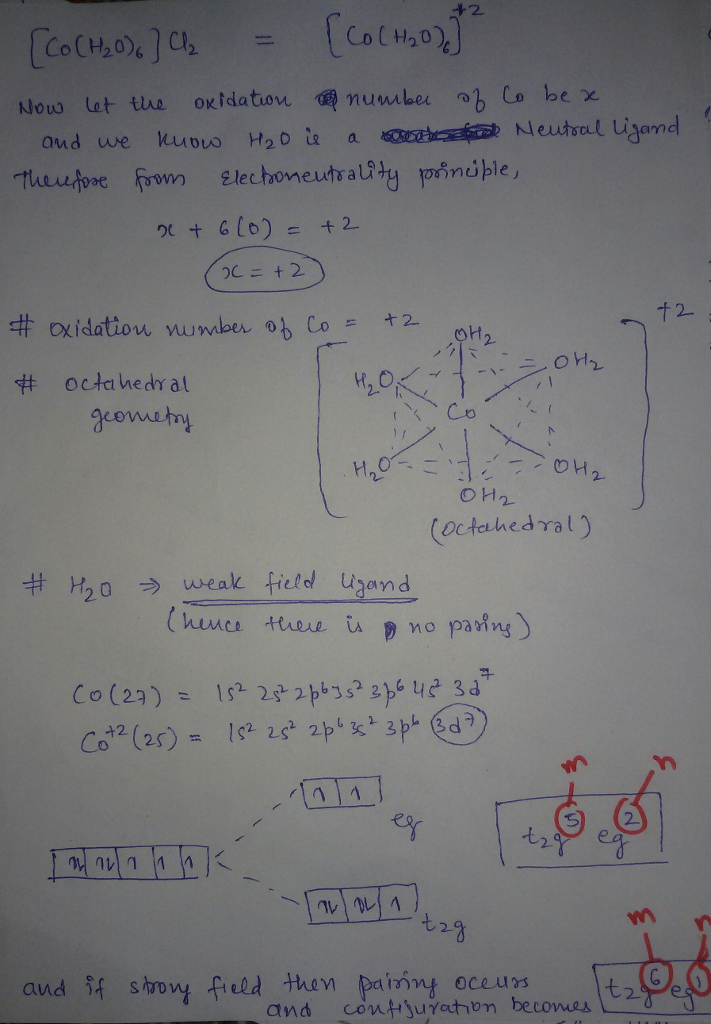 but H2O is weak fiels ligand hence configuration with weak field is TRUE
but H2O is weak fiels ligand hence configuration with weak field is TRUE
I had given you configuration as a strong ligand just because if you assumed it to be strong field. otherwise h20 is weak field ligand
So much stress and so little time? We’ve got you covered. Get your paper proofread, edited or written from scratch within the tight deadline.


